We are developing new interventions for neurological diseases of aging by leveraging the biology of human aging
We disrupt the status quo by utilizing cutting-edge technology, such as artificial intelligence and machine learning, overcoming roadblocks where others see limits. This approach drives faster market entry, lower costs, and accelerated value creation

LathMized™ Green Platform Technology
Our green platform technology optimizes the bioavailability and stability of new molecular entities, existing drugs and supplements using sustainable processes. It eliminates the use of toxic chemical cocktails, waterless process, minimizes energy usage, and ensures no trace contamination
Harnessing Cellular Health to Transform Neurological Aging
Neurodegenerative diseases are some of the most urgent health challenges today. Every few seconds, someone is diagnosed with a life-threatening disease like Alzheimer’s, Parkinson’s, ALS, Multiple Sclerosis, or FSHD, a rare type of muscular dystrophy
Declining cellular health is a critical risk factor, and Bryleos is at the forefront of addressing these issues by leveraging advanced NAD+ therapeutics to target multiple factors of neurological aging
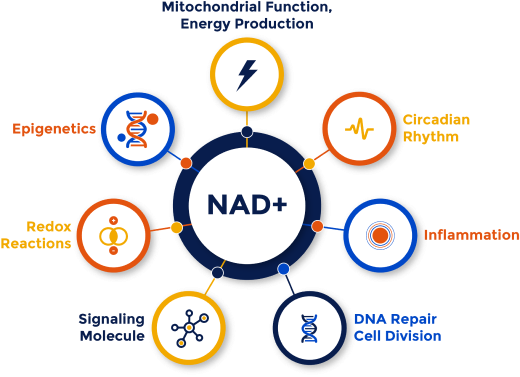
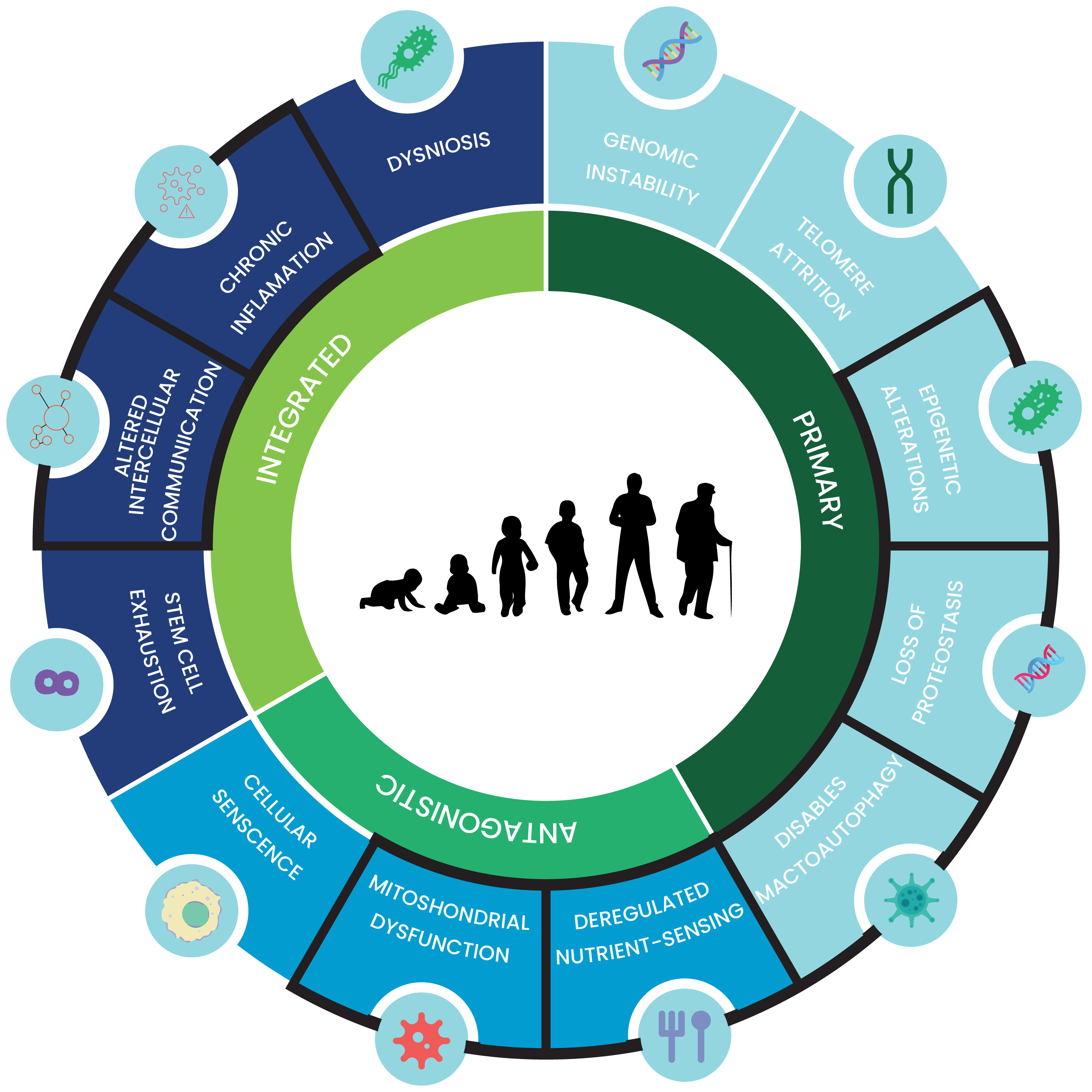
BRY101 is Engineered to Impact Multiple Hallmarks of Aging
BRY101, our lead program, is a first-in-class oral therapeutic targeting mitochondrial dysfunction, neuroinflammation, and oxidative stress. Unlike traditional single-target drugs, BRY101 modulates key pathways involved in neurodegenerative disease progression, offering a breakthrough approach to treating age-related neurological conditions